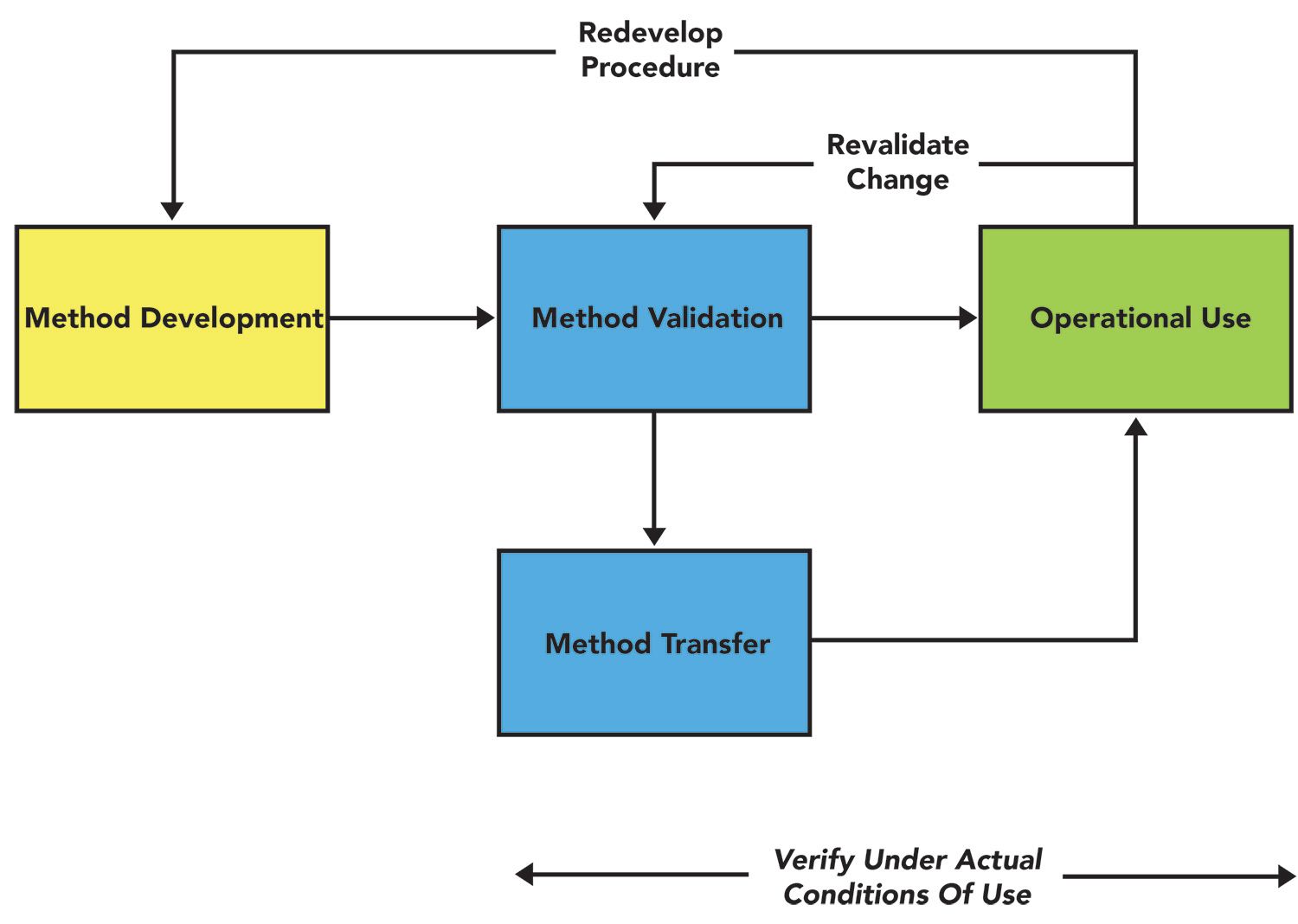
The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content.

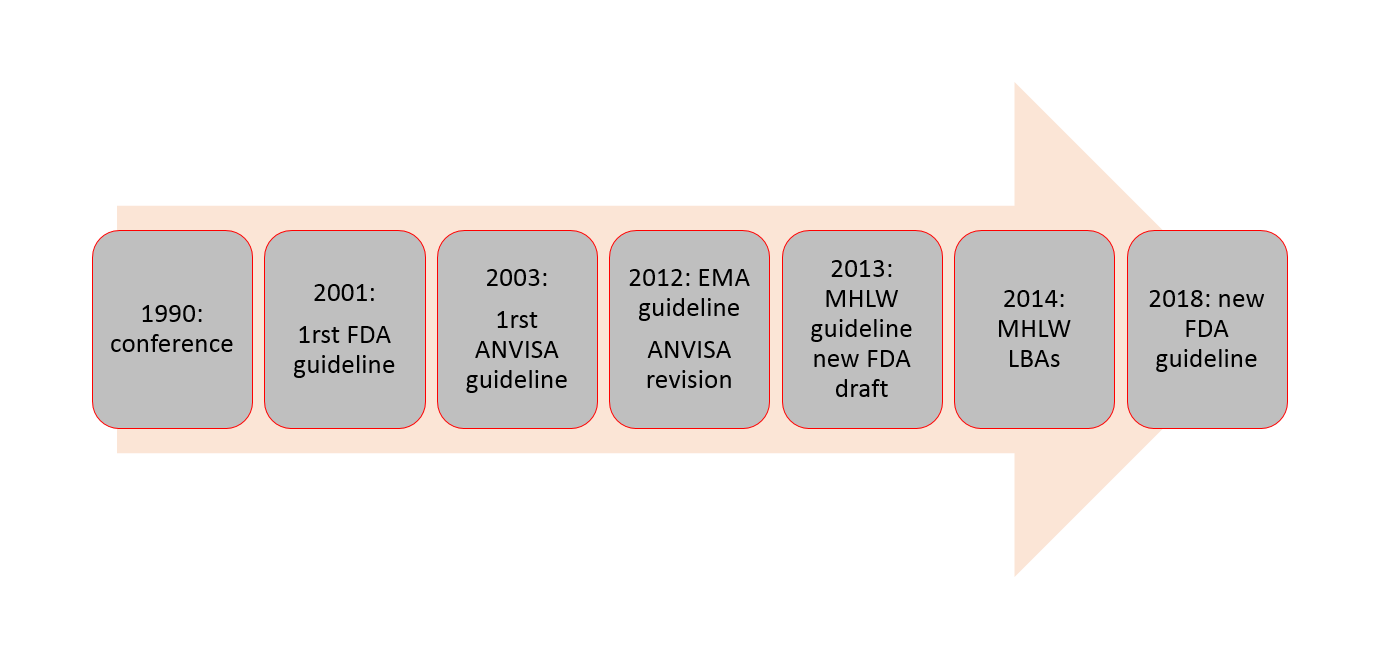
PDF) M10 EMA Guideline on Bioanalytical Method Validation (2011) FDA Guidance for Industry: Bioanalytical Methods Validation (2001) → revision (2018) MHLW Guideline on Bioanalytical Method - DOKUMEN.TIPS

Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry - ScienceDirect
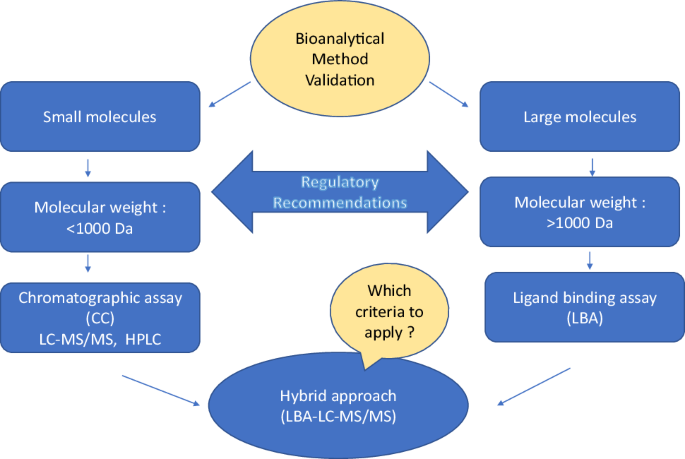
Review of Recommendations for Bioanalytical Method Validation: Chromatographic Assays and Ligand Binding Assays | SpringerLink
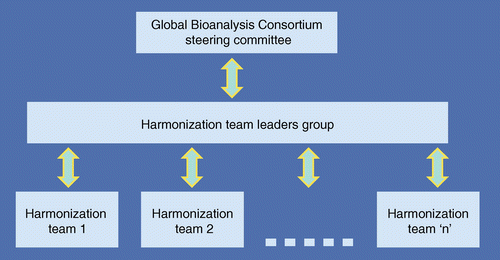
Building the Global Bioanalysis Consortium – working towards a functional globally acceptable and harmonized guideline on bioanalytical method validation | Bioanalysis
![PDF] omparative assessment of bioanalytical method validation uidelines for pharmaceutical industry | Semantic Scholar PDF] omparative assessment of bioanalytical method validation uidelines for pharmaceutical industry | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ec1710368980e1a3dcbcc921fb7062d9bb753191/6-Table4-1.png)
PDF] omparative assessment of bioanalytical method validation uidelines for pharmaceutical industry | Semantic Scholar
![White Paper] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC) - CMIC | Pharmaceutical Development Services (CRO, CDMO, White Paper] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC) - CMIC | Pharmaceutical Development Services (CRO, CDMO,](https://en.cmicgroup.com/wp-content/uploads/2019/11/featuredimage_recommendationsontheinterpretationofnewEMAguidelines-218x300.png)
White Paper] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC) - CMIC | Pharmaceutical Development Services (CRO, CDMO,

PDF) The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content | Peter van Amsterdam - Academia.edu

Metabolon, Inc. on Twitter: "Three levels of biomarker validation: Exploratory, non-GCP, & GCP. Our capability to adhere to GCP/GCLP is in accordance w/ FDA bioanalytical Method Validation or EMA Guidelines on Bioanalytical
![PDF] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC). | Semantic Scholar PDF] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC). | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/cb32cb17c42115291ad9af2bb1eedea9761db28c/2-Table1-1.png)
PDF] Recommendations on the interpretation of the new European Medicines Agency Guideline on Bioanalytical Method Validation by Global CRO Council for Bioanalysis (GCC). | Semantic Scholar
The EMA Bioanalytical Method Validation Guideline: process, history, discussions and evaluation of its content.

EMA released for public consultation the draft ICH guideline M10 on bioanalytical method validation | EPTRI